Centralize specs, eliminate duplication, and win faster item-master approvals and tenders across every variant and market.
.avif)
Build a single source of truth for UDIs, IFUs, images, specs, bill of materials, kits, and translations. Replace scattered sheets and siloed PLM, ERP, and DAM tools with one catalog your teams trust. Marketing, sales, QA, and regulatory work from the same record, so provider requests, tenders, and GPO updates get answered on the first pass.

Buyers never have to point out what’s missing. Quality Reports reveal gaps in attributes, claims, sterilization methods, or languages and make bulk updates simple. Product families and sizes reach higher completeness, hospitals see fewer item-master rejects, and ecommerce and distributor portals benefit from enriched, consistent content. IFUs, images, and translations stay accurate and up to date.
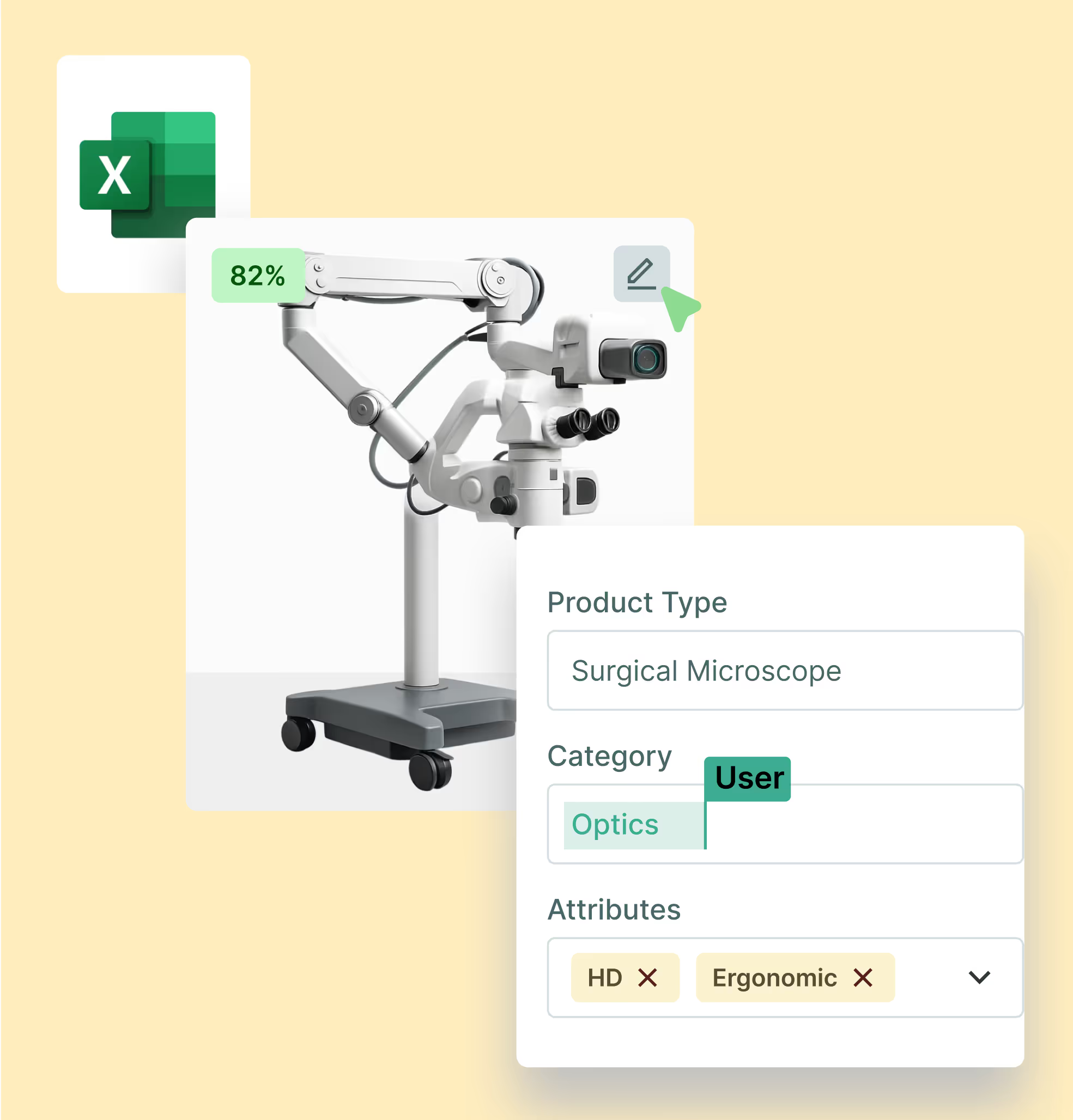
Device teams run ERPs, QMS, eCommerce, CPQ, and service apps that rarely speak the same language. Sales Layer’s PIM syncs product truth across them, reducing rekeying and integration errors. Push clean attributes and digital assets to marketplaces, ecommerce, and procurement platforms such as SAP Ariba or GHX , so every team works from the same catalog and updates flow without busywork. Cut the back-and-forth on spreadsheets, and ensure contracts and assortments stay aligned everywhere you sell.
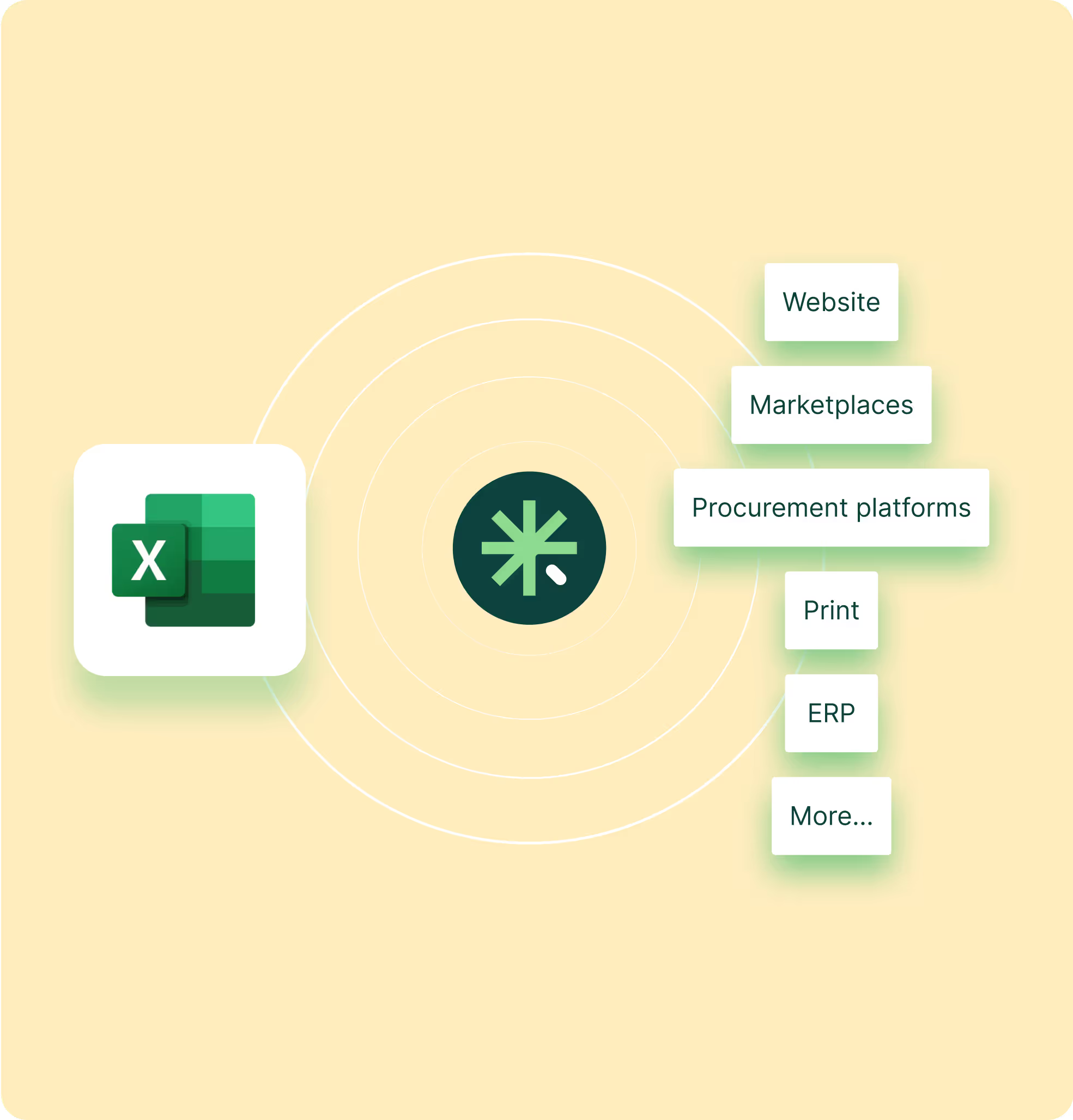
New SKUs stall when teams chase specs, update PDFs, and recheck packs across languages. With a single hub for attributes, media, and documents, you update once and publish to web, distributor portals, and item masters. Cut catalog refresh time, shorten Value Analysis cycles, and move from idea to market with fewer emails and rework. Map fields for GUDID or EUDAMED and keep variants aligned by region.

%20(1).png)



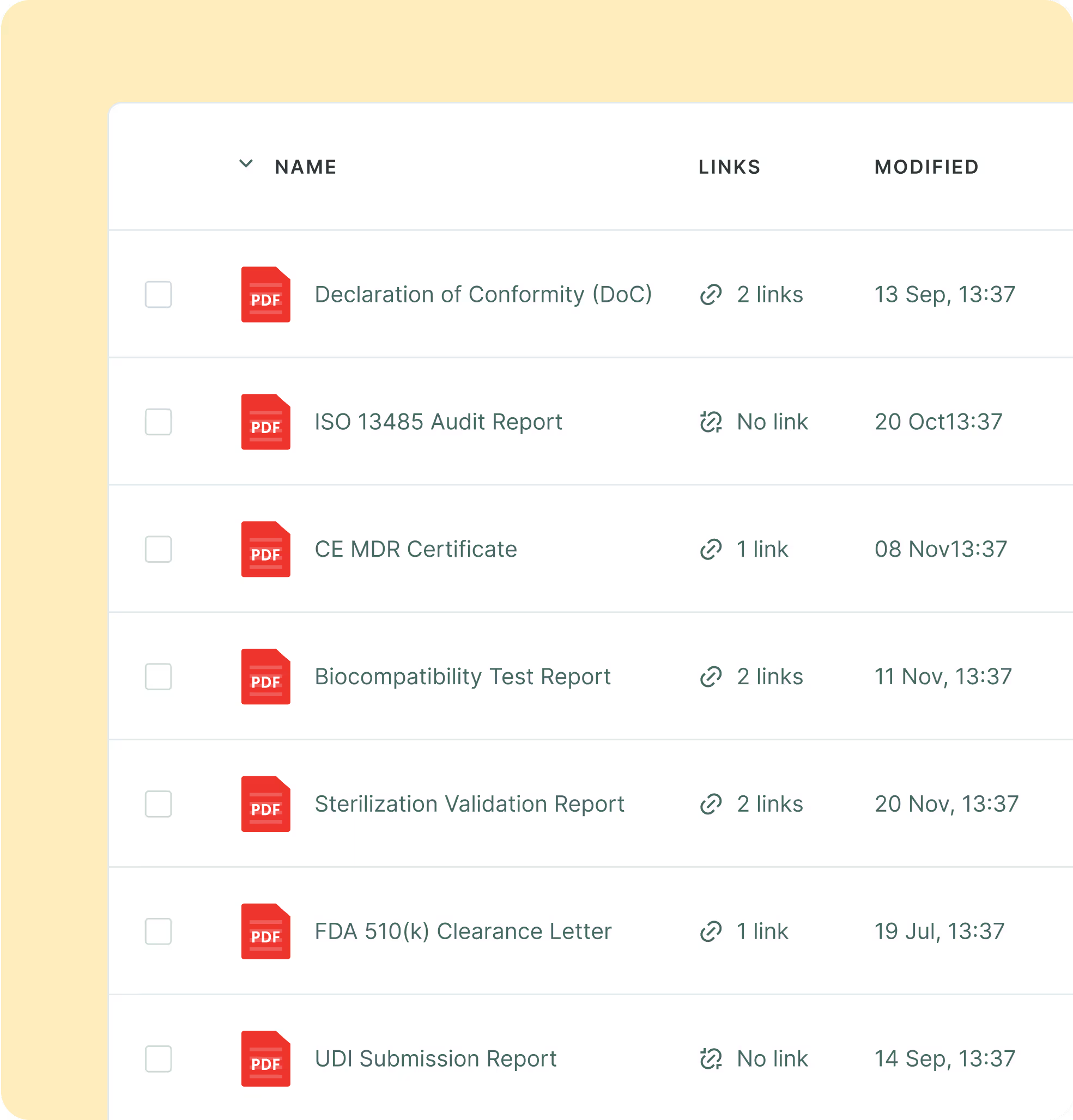
Regulators expect current labels, IFUs, and declarations by market. Managing these by hand risks outdated files and audit findings. With Sales Layer, work within your ISO 13485 QMS, store the latest approvals, map UDI-DI to SKUs, and link assets to packaging levels. RA/QA get full history and version control, and sales get the right document pack per country when they need it. Support MDR and 21 CFR 820 workflows with clear ownership.
Reps and channel partners need quick access to specs, images, IFUs, and compatibility charts to quote and recommend safely. Give them searchable catalogs, up-to-date spec sheets, and variant finders. Fewer back-and-forths, faster quotes, and fewer product questions during trials and evaluations in the operating room or lab. Power CPQ with accurate options and give GPO portals current content.
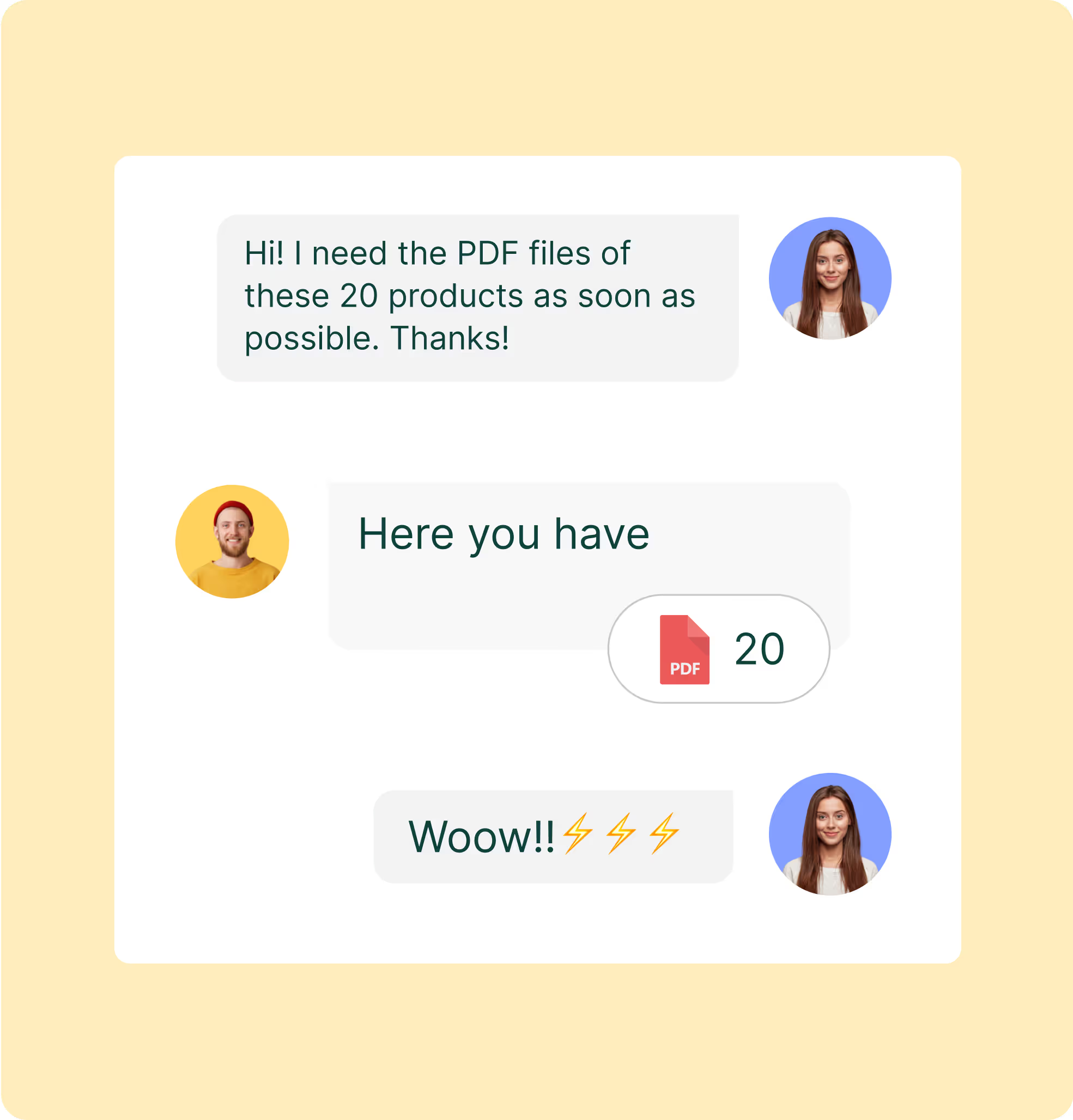
Hospitals want clean item masters and tender packs. Sales Layer generates buyer-specific exports with the right units, regulatory fields, and language sets. Create PDFs with accessories and compatible components, then share a link that always points to the latest approved version with procurement and biomed. You cut manual formatting, reduce errors, and help buyers source the right device the first time.
.avif)
Rosie Walden
Digital Marketing & Communications Specialist at Unigloves (UK)
